Could Columbus help find COVID cure?
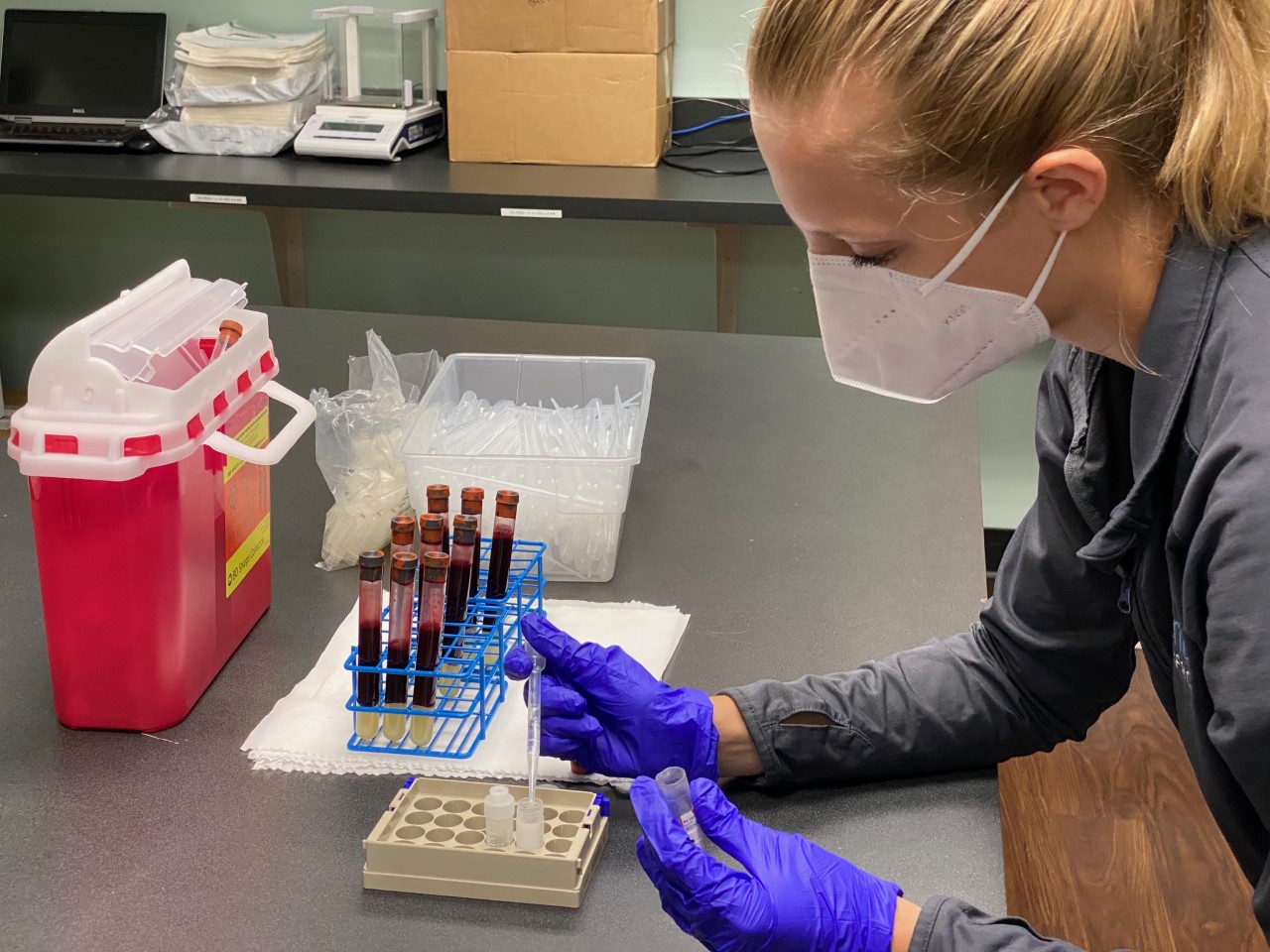
Aventiv Research is conducting a new COVID-19 clinical trial in Columbus, Ohio, and unlike some of the vaccine trials that have recently been announced, this test requires volunteers who have already tested positive for the virus.
Volunteers will be randomly assigned to either receive a placebo or an antibody treatment designed by drugmaker Eli Lilly through a one-time IV administration.
Early laboratory studies have shown that the treatment neutralizes COVID’s ability to infect cells and replicate.
To test this in humans, Aventiv Research is reaching out for volunteers who are willing to sign up within three days of testing positive. Additionally, they must have one or more mild or moderate COVID-19 symptoms, including: fever, cough, sore throat, headache, muscle pain, nausea, abdominal pain, diarrhea, or shortness of breath when active.
BROUGHT TO YOU BY
Study participants will be seen at Aventiv’s main clinical pharmacology site at 99 N. Brice Rd., Suite 260. After the treatment or placebo is administered, volunteers will have their health monitored through a series of physical exams–blood samples and nasal swabs will be taken to measure levels of virus.
Aventiv also notes they are taking steps to protect patients. They have modified their research center with touchless entry through a private entrance, and there’s a dedicated patient exam room and restroom, which will receive electrostatic cleaning between visits. Clinical staff will change and decontaminate in transition rooms following exams.
Ohio reported 1,453 new coronavirus cases on Tuesday—the highest one-day total since the end of July.
Compensation for time and travel up to $825 dollars may be available to those that qualify. Those interested in participating should call (614) 501-6164 or visit AventivResearch.com to learn more.
BROUGHT TO YOU BY




